New Course at WCU – Spring 2022
Design of Medical Devices
Martin Tanaka, PhD, PE
Course Description: Design of Medical Devices is a special topics course which provides an overview of several important biomedical engineering topics. It includes the processes used to design and develop commercial products, biomaterials and their applications in medical devices, and content to understanding regulatory requirements. It also includes elements of human anatomy and physiology, engineering calculations, human subject testing, and business and ethical considerations related to the topic. Credits 3
Prerequisites: Materials Science, Computer Utilization, Mechanics of Materials, Probability and Statistics, and Ordinary Differential Equations
Course Aims and Objectives: This course is a technical elective for students who have completed their fundamental training in mathematics and basic engineering courses. The course is designed for a junior or senior in mechanical engineering but may be suitable for students in other majors who meet the required prerequisites necessary to be successful in the course. Students will learn fundamental information about biomedical engineering through lectures and interactive classroom discussions. Several group projects will be used to address challenging material and foster collaborative effort essential in this interdisciplinary field.
By the end of this course, students will; Develop foundational knowledge in commercial product design; Develop a fundamental understanding of basic human anatomy, mechanical properties of bones and joints, and the ability to calculate stress in a biological system; Understand how biomedical devices are developed and the federal regulations that control the development and sale of these products; Read technical articles in the field of biomedical engineering and describe the content contained within.
Summary of Course Topics
Commercial Product Design Topics: Voice of the Customer; Product Specifications; Conceptual Design; Initial Product Design; Design Optimization; Design Review; Prototyping and Testing; Problem based learning; Critical Thinking in Engineering; Human centered design; Divergent Thinking
Biomaterials & Medical Devices Topics: Introduction to biomaterials; Biocompatibility; Hydrogels; Tissue Engineering Fundamentals; Scaffolds, cells, biologically active molecules, and bioprinting; Adult stem cells, embryonic stem cells, induced pluripotent stem cells (IPSC); Therapeutic cloning & controversy; Chemical signals, autographs, allographs, xenografts, synthetic tissue grafts, transplant rejection; Human Structural Anatomy – bones, ligaments, cartilage, tendons, biomechanical properties; Musculoskeletal System – muscles, joints, etc.; Prosthetics and Orthotics, and Exoskeletons; Artificial vision devices; Cardiovascular system Part 1 – Anatomy of the heart, and flow through arteries and veins, Pressure gradients and osmotic pressure in capillary beds, Oxygen partial pressure, diffusion, equilibrium, and hemoglobin; Cardiovascular system Part 2 – Blood pH, CO2, and bicarbonate, aneurisms and strokes, balloon angioplasty and stents
Regulatory Science & Ethics topics: Origins of the FDA; Product Codes, Device Classes, Exempt, 510k, PMA, etc.; Guest Speaker from Industry; Guest Speaker From FDA; Business Aspects in BME (Product Dev. Process, CGMP, Reimbursement, V&V 40); Human Subject Research – biomedical engineering ethics, Institutional Review Boards; Data Analysis – review of statistics, t-test, ANOVA; Course Review Discussion and integration of concepts
Module 1: Design and Development of Commercial Products
Commercial products are design and developed using a structured process. Medical devices are no exception. Some of the benefits of using a structured process include the following: Reduced risk of product failure; Lower overall product development costs; Faster time to market; Alignment of engineering and business objectives; Lower manufacturing costs; Higher product quality
Thus, it is not surprising that major manufacturers use this process. Starting with the business roadmap and working around clockwise the process includes setting up a team, understanding the needs of your customers, developing engineering designs, optimizing and refining the designs, prototyping, testing, preparing for manufacturing, and finally production.

Mechanical Engineering Design
Many people don’t know how to design a mechanical system using engineering. Here is a simple breakdown. It’s not the only way and your approach could differ based on the type of product you are engineering. But if you have limited experience, it may help you get started.
- Begin by thinking about the functions that your product needs to satisfy. Consider different approaches / ways to solve each problem and come up with an initial design.
- Begin designing the product from the inside out. Focus on the interactions of the individual components, motors, gears, belts, buttons, etc. Model the parts in 3D CAD and assemble them together. Optimize the design to avoid long shafts under bending loads or other areas that induce high stress (no math yet), and optimize the part configuration for a compact design.
- Take measurements of your assembly and begin to design the housing that will hold it. Focus on the internal geometry needed to properly hold your important functional parts, not on the outside “box”. In good mechanical design the outer housing will often look like you poured a thick layer of cement over the functional parts. Add feet for mounting and clean up the aesthetics.
- Now that the design concept is solidified, its time for the engineering. Use the geometry in the model to obtain dimensions for your calculations. Look for areas of high stress, calculate it, and compare it to the material strength. If it is less, move to the next area. If it is more, modify the geometry or the material and repeat the analysis. If it is far lower, consider reducing the size to reduce cost and weight and run the analysis again. This process is the key to engineering. The goal is to solve problems through calculation, not trial and error.
- Once you have checked everything that you can think to check, it’s time to verify your work. Build a prototype and test it. This can take a long time and be expensive, so hopefully you will only need to do this once.
- Assuming that the design passes the tests, you can now begin building production tooling and manufacturing equipment to make your product.
Human Centered Design
In human centered design, emphasis is placed on people rather than the product. It includes meeting with the end users of the product and getting to know them before the product is designed. Making quick prototypes and placing them in the hands of the customers to get feedback. Iterating and optimizing toward a final solution. https://youtu.be/musmgKEPY2o

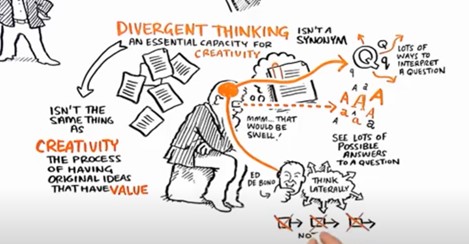
Divergent Thinking
Divergent thinking enables people to discover new ways to solve problems. Unlike convergent thinking where possibilities are narrowed down until only one idea remains, divergent thinking aims to spread out our thoughts. Thinking in new ways can reveal new solution approaches. Good used of this method in product design includes first thinking divergently to explore the design space, then thinking convergently to identify a solution approach that satisfies the requirements. https://youtu.be/BHMUXFdBzik
Module 2: Biomaterials and Medical Devices
The field of biomaterials is over 70 years old with some of the first medical devices being used in the late 1940s and early 1950s. Medical devices have had a significant impact on human health and the economy, saving millions of lives and improving the quality of life for millions more.
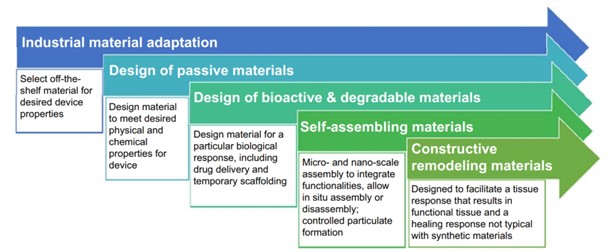
Biomaterial – A biomaterial is a nonviable material used in a medical device intended to interact with biological systems. The original biomaterials were “off the shelf”. These were standard materials like stainless steel developed for other applications. Using them for a medical application made them “biomaterials”. Next came passive materials specifically design to improve biocompatibility. An example is 316L, surgical stainless steel. It’s a modified version of 316 stainless steel that has low carbon content to further enhanced corrosion resistance. Having found ways to minimize adverse reactions, the next phase of biomaterials was to enhance positive interactions. Coronary stents coated with drug releasing chemicals not only keep blood vessels open, but also provide medication directly to the inside of the vessel. The latest innovation in biomaterials include self-assembling structures and materials that facilitate functional remodeling.
Biocompatibility – For a material to be biocompatible it must have the ability to perform with an appropriate host response in a specific application. Examples of “appropriate host responses” include resistance to blood clotting, resistance to bacterial colonization, and normal uncomplicated healing. Notice that it is specific to the application, so the same material may be biocompatible when used as a skin patch, but not biocompatible when used as a contact lens or an implanted tissue mesh.
Biological Biomaterials – Solid porous scaffolds infused with cell and drugs can be used to develop regenerated tissue. This tissue can be developed within a bioreactor (in vitro – outside the body) or within the body (in vivo). Fibrous scaffolds, decellularized scaffolds, and hydrogels may also be used

Medical Devices
Prosthetic Heart Valves – 4.5 million replacement valves are implanted each year worldwide. They may be mechanical valves such as the bileaflet tilting disk or made from biological tissue such as a xenograft from a pig.
Total Hip Replacement – Mechanical stress, degenerative diseases, overuse, or natural aging can lead to joint dysfunction. Over 300,000 total hip replacements are performed in the USA each year. Many people can walk a few days after surgery and resuming athletic activities may also be possible.
Ventricular Assist Device – Nearly 5,000,000 Americans are living with seriously failing hearts. Yet, there are only about 3,500 donor hearts available in the US each year. VADs may be used to prolong and improve the quality of life.
CT Scanner – Some medical devices are used for diagnosis. A computerized tomography (CT) scanner combines a series of X-ray images taken from different angles around your body and uses a computer to assemble a 3D image of your bones and other radiopaque materials.
Module 3: Understanding the regulatory requirements
The information contained below was generated from CDRH learn on the FDA website. https://www.fda.gov/training-and-continuing-education/cdrh-learn



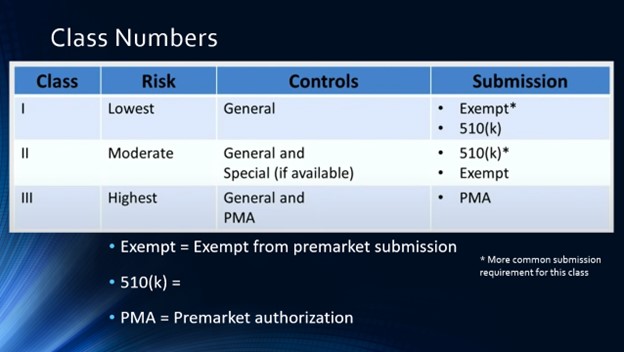







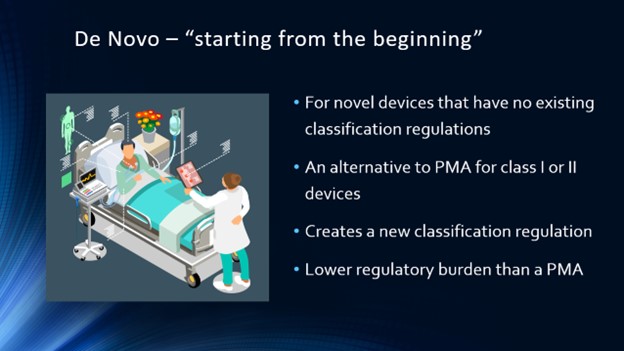


Faculty are encouraged to contact me directly to obtain detailed information about syllabus, lectures, assignments, projects, and exams. Verification of credentials is required.
Martin Tanaka received support from the National Science Foundation (Award #2149517) that contributed to this work.

